Biomimetic in vitro systems
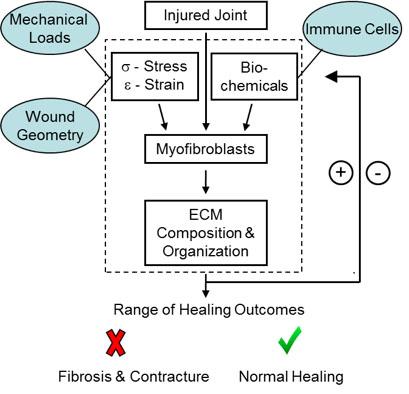
One of our main interests is in using ideas from tissue engineering and multi-scale mechanics to engineer in vitro models of tissues. Two particular applications of interest are with respect to cutaneous wound healing and scar formation and to traumatic joint injury and contracture.
We have developed several 3D in vitro platforms for observing and quantifying dynamic cell-cell and cell-matrix interactions that arise during the remodeling process in response to both mechanical and chemical cues. These systems typically consist of fibrin and collagen gels embedded with cells isolated from fibrotic and non-fibrotic tissue, such as rabbit joint capsule fibroblasts (RJCFs). Time-lapse images acquired at multiple positions are later analyzed to provide quantitative data on cell force generation, migration, collagen production, and other ECM remodeling processes over a wide field of view. At the end of the experiment, the gels are then extracted for qPCR, histology, or protein quantification.
Mechanobiology
In addition to biochemical cues, mechanical cues inform and drive cellular behavior. These cues can from the geometry and material properties of the local environment, or from forces transmitted through the tissue from the outside surroundings, or from traction forces exerted by neighboring cells.
Drug delivery
Multi-scale mechanical interactions
Multiscale mechanical interactions are scale-spanning physical interactions between cells, the extracellular matrix (ECM), and the tissue, and they are critical to all phases of a tissue’s life cycle (i.e., development, growth, homeostasis, aging, and disease). These dynamic and reciprocal interactions are inherently complex and produce emergent behaviors that cannot be easily understood using reductionist approaches. It is therefore essential that a theoretical framework exists that can incorporate data and observations from different experiments into a unified picture so that basic principles of mechanobiology can be understood and used to direct tissue remodeling.
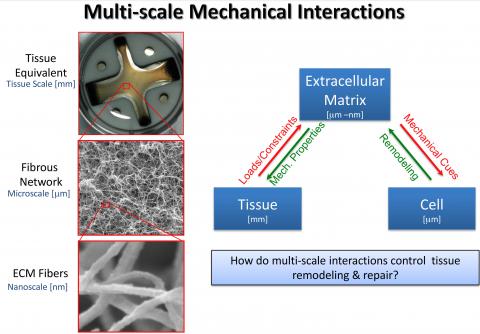
- Tissues function on the mm and cm scale but are composed of proteins and cells that organize into structures that span multiple scales.
- For example, in the cell compacted collagen gel (tissue equivalent) depicted, the mechanical properties of the gel are dependent on the heterogeneous organization of collagen fiber networks, each of which is composed of cross-linked individual collagen fibers.
- When loads and constraints are applied to the tissue, the forces are transmitted down through the extracellular matrix (ECM). These mechanical cues can direct local ECM remodeling by resident cells, which in turn changes the local mechanical properties of the tissue.
- The global tissue properties also change, which alters the distribution of forces and the presentation of mechanical cues at the cellular level. These processes continue across scales until equilibrium is achieved.
- Our group is interested in understanding how such interactions drive local tissue remodeling and repair.
Synthetic biology
Synthetic biology is an area of interest for the group that has been driven by undergraduate interest in the annual iGEM competition.